HPLC Troubleshooting - Peak Shape Problems & Ghost Peaks
In daily work with HPLC systems, we meet a variety of problems in chromatograms, like baselines, peaks, retention time, etc. Here we analyze some problems about the peaks.
1. Small Peak
2. Negative Peak
3. Tailing Peak & Leading Peak
4. Double Peak Phenomenon
5. Peak Splitting
Small Peak
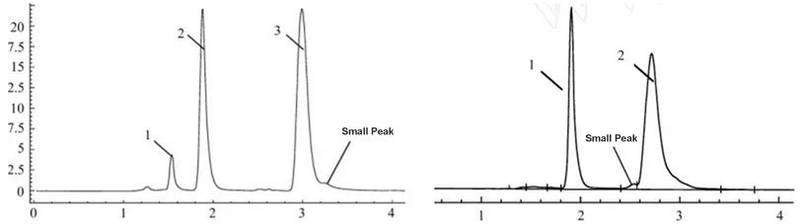
- The sample is not pure
- Using different mobile phase and HPLC columns to separate and compare samples, and select suitable separation conditions.
- Column collapse of analysis column or protective column
- This is a common situation. Change the analysis column or guard column, then compare the peak shape.
- Peak splitting tends to occur in all peaks with column head collapse. Regeneration and cleaning of the HPLC column will improve the separation effect.
- Column capacity decrease
- After a long time of usage, there are some strong retention components adsorbed in the column, and small injection volumes tend to have peak splitting. The problem can be ameliorated by cleaning the column with a solvent with strong elution ability or by just replacing the column.
- The sample solvent does not match the mobile phase or the injection volume is too large
- When the polarity of the sample solvent is larger than that of the mobile phase, sometimes the peak deformation and splitting phenomenon are easy to occur even if the sample volume is small. It is recommended to dissolve the sample with the mobile phase.
- Improper mobile phase
- This situation is rare. Some samples under specific chromatographic conditions may have a dynamic equilibrium structure, and the double peak, the double peak can not be separated completely. Change the chromatographic conditions, especially the pH value to make the peak shape normal.
- Sample decomposition
- Unstable samples will be changed into other substances during chromatographic separation and double peaks occur. So using the sample treatment methods or change chromatographic separation conditions.
Negative Peak
- The mobile phase absorption background value is too high. Change the detection wavelength appropriately.
- Air is entered during the injection. Using exhaust treatment. Re-inject when the baseline is stable.
- The absorption of sample components is lower than that of the mobile phase. Change the mobile phase or the detection wavelength.
- The solution of the sample preparation is different from the mobile phase. Reprepare the sample with the same solvent as the flow, or dilute the sample.
Tailing Peak & Leading Peak
Tailing Peak
- Interference peak. Separation under optimized conditions.
- Column collapse. Replace column.
- Mobile phase pH is not appropriate. Adjust the pH value. Add additives to eliminate secondary effects (interaction between components in the mobile phase and the column)
- Improperly connected pipeline. Large dead volume. Reconnect the pipeline.
- The injection volume is too large. Reduce it.
Leading Peak
- Improper solvent. Choosing the appropriate solvent.
- The sample is overloaded. Reduce the injection volume.
- The column temperature is too low. Raising the column temperature.
- Column damaged. Replace column.
The main reason for the tailing peak and the leading peak is the improper selection of the mobile phase, which can be better improved by adjusting the polarity of the mobile phase or adding acid appropriately. Generally, acid and base in the mobile phase have a great influence on the tailing peak and the leading peak. The leading peak may be caused by column overload, and the tailing peak may be caused by sample contamination. Choosing the appropriate mobile phase and adjusting the pH value will improve this situation. Tailing peak is related to the column, maybe overload, dilute the sample, or use a new column. Sometimes the tailing peak is caused by the unseparated impurities with similar organic properties. You may need to optimize the analysis method or replace the column. Tailing peaks may also cause by column effect decline or column collapse after long column use time. Sometimes, tailing peaks are related to the properties of the sample, which some chemicals need to be added in the mobile phase to optimize the peak shape (depend on the specific situation).
Double Peak Phenomenon
1. Column
If every peak has a double peak in the sample analysis process (the shorter the appearance time, the less likely it is to have a double peak), especially the analysis of the pure sample, there is a problem in the column (column head damaged, stationary phase in the column head dirty or lost).
If the sample injection volume is small and the column works well, the peak is a large peak with a small peak, it is not trailing. This may be caused by the column block. Wash the column with mobile phase solvent or acid cleaning or other solvents in a reverse direction, the block in the column will be washed away. Forward direction washing sometimes also can solve this problem.
If the peak shape is trailing with little difference between two peaks, it may be caused by the dirty or loss of the stationary phase in the column header.
2. Solvent polarity and injection volume
For reversed-phase chromatography, the common mobile phases are methanol, acetonitrile, and water. Other additives are added to improve the separation performance. The sample is generally dissolved in a soluble solvent with the mobile phase, and the best solution is to use the mobile phase. When the solvent is strong polarity reagent (such as pure methanol, acetonitrile, pure ethanol) and the analysis system is mainly water, the double peaks phenomenon will occur with a large injection volume of pure sample (such as quantitative tube for 20ul): a peak combine with a smaller peak (different every time) with a tail, the retention time (relative to less sample quantity) will be earlier. If the injection volume is reduced by more than half, the peak shape will return to normal. This is because the solvent polarity of the sample is too different from that of the mobile phase, the mobile phase has no time to dilute it to reach equilibrium.
Another reason is that the injection volume is not necessarily large, but the absolute amount is large, and the two peaks on the chromatogram are close together, basically at the same height, without trailing (if the peak appearance time is very short, it may also be a column problem). If it is caused by a large injection and overload column, dilute the sample before injection.
3. Sample characteristics
Some samples, due to the characteristics of their chemical structure, have the phenomenon of tautomerism, and the tautomerism can not be separated, but in a dynamic equilibrium existence. In the chromatographic analysis, under a specific condition, a substance will appear double peaks. These double peaks are very close with the same height and no tail. The double peak phenomenon will disappear when the condition is changed (especially pH).
4. Device parameters
The parameters of the record are generally set internally and do not need to be modified without special needs. The HPLC recording time is generally 5ms. If the recording interval is shortened, one peak will become two or more peaks.
Ghost Peak
- The system was not injected. Leaking or the sample isn't soluted.
- The sample isn't handled properly. The sample is damaged or derivatized.
- The UV detector is not sensitive.
- Residual substances are found in the injection valve and instrument pipeline.
- The mobile phase is contaminated.
Ghost peak is no relationship with the previous sample. Ghost peaks also appear with blank samples. The retention time may differ from sample tests.
This is caused by the previous sample residue. Small peaks that are similar sample peaks will appear.
Peak Splitting
- Column contamination
- Frit block
- Column head collapsing (liquid phase pH≥7). Choose special columns will solve it.
- Solvent effect. The solvent is more polar than the mobile phase. It is also decided by the sample injection volume.
- Bubbles in the detector
Tian Jing
Manager & Engineer in GALAK Chromatography. Master of Chemical Engineering.
During my college study, I found liquid chromatography to be a profound subject. I know the painful struggle a novice needs to go through to get started. I share this article to help you solve your problems quickly.