Affinity Phases Selectivity
Affinity phases offer the highest specificity and selectivity in biomolecular separations and purifications. Purifications up to several orders of magnitude can be achieved in a single step. Affinity separations can often remove contaminants difficult to eliminate using conventional chromatographic procedures.
Mechanism
The basis of affinity chromatography is a ‘lock and key’ type mechanism. An affinity ligand, specific for a binding site on the target molecule, is coupled to an inert chromatography matrix. Using suitable binding conditions, target molecules are bound to the affinity ligand according to its specificity. Unbound solutes are washed through the column. The adsorbed target molecules are then desorbed and eluted from the column. Purification of several thousand-fold may be obtained due to the high selectivity of the affinity interactions.
Group-specific affinity resins (eg. Tosoh Bioscience) bind molecules sharing specific structural features. Alternatively, if greater specificity is required, ligands with precise specificity for the target molecule can be used. For example, the Protein A phase has a specific affinity for the Fc region of immunoglobulins.
Immunoaffinity Chromatography
Immunoaffinity chromatography is a specialized form of affinity chromatography,
utilizing an antibody or antibody fragment as the ligand immobilized on to a
solid support in such a manner that its binding capacity is retained. Figure 1 shows a schematic diagram of the stages involved in a typical immunoaffinity analysis: loading the sample, washing the column to remove matrix components and impurities and eluting the target compound.
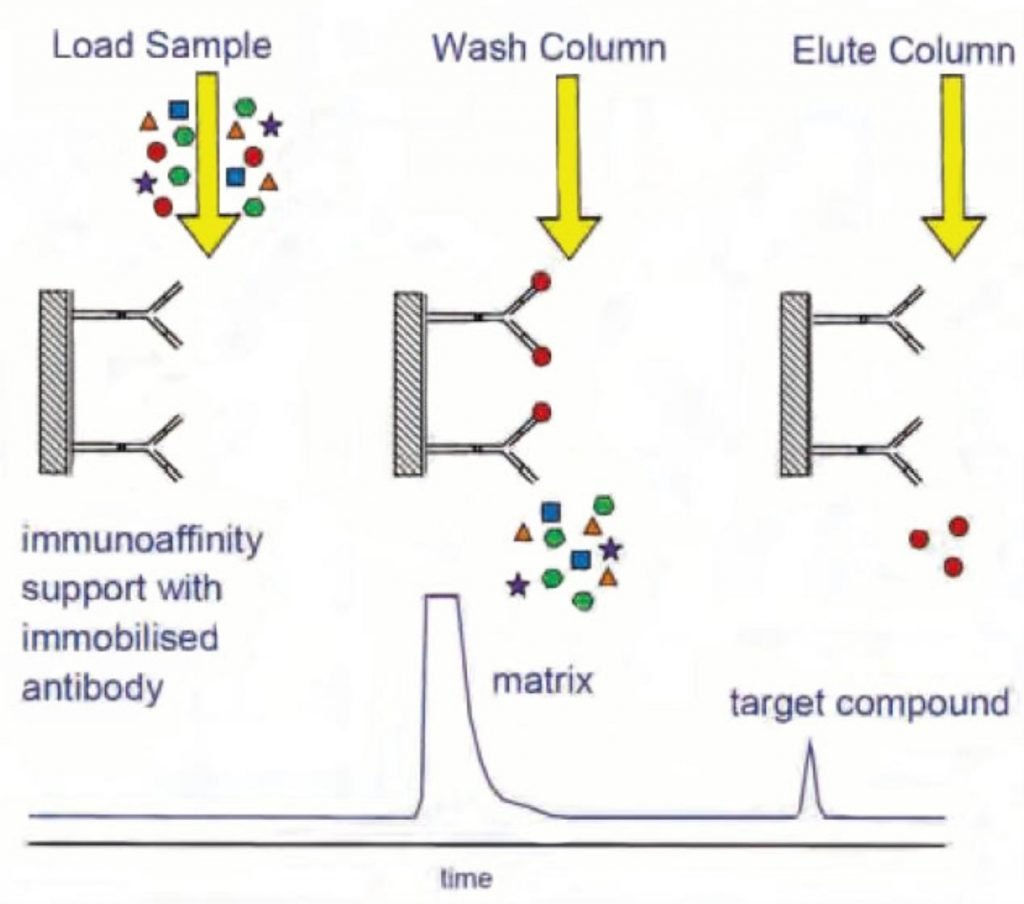
Applications
Affinity and immunoaffinity chromatography techniques are applicable in a variety of disciplines including biochemistry, immunochemistry, virology, and molecular biology. Due to the increasing availability of a variety of antibodies, separations based on immunoaffinity techniques are being increasingly used in a wide range of applications involving the purification of complex biological samples.
The main application areas of immunoaffinity chromatography are proteins and enzymes. Method selectivity can be enhanced by combining the pre-concentration and pre-treatment of samples offered by immunoaffinity phases, with the separation capabilities of reversed-phase HPLC. The technique can also be used prior to MS analyses in proteomics.
GALAK Affinity Columns & Bulks
Analysis Column Absolut A
| Base Material | Particle Size | Flow Rate | Life Service* | pH range | |
| Absolut A | Polymer | 30um | 0.5-3 ml/min | 3000 circles | 2-10 |
| Absolut A Pro | Polymer | 30um | 0.5-3 ml/min | 6000 circles | 2-10 |
*Life Service is based on analysis conditions.
Sepromax® A40 plus & GLK gel PrA/G Affinity Phases
| Base Material | Particle Size | Flow Rate | Capacity | pH range | |
| Sepromax® A40 plus | Polymer | 40um | 0.5-3 ml/min | 40 mg human IgG | 2-10 |
| GLK gel Pr A | Agarose | 90um | 0.5-3 ml/min | 20 mg human IgG | 2-12 |
| GLK gel Pr G | Agarose | 90um | 0.5-3 ml/min | 20 mg human IgG | 2-8 |
| GLK gel Ni-IMAC | Agarose | 90um | 0.5-3 ml/min | 30 mg Myoglobin | 3-12 |

Tian Jing
Manager & Engineer in GALAK Chromatography. Master of Chemical Engineering.
During my college study, I found liquid chromatography to be a profound subject. I know the painful struggle a novice needs to go through to get started. I share this article to help you solve your problems quickly.
Does this article still not solve your problem?
Contact us now! We are 7/24 available.
As manufacture for liquid chromatography products, we provide
